Battery
The battery stores electrical energy and supplies power to the vehicle electrical system. In modern vehicles, the battery is not just needed to start the engine. It must also supply power to a large number...
Function
The battery stores electrical energy and supplies power to the vehicle electrical system. In modern vehicles, the battery is not just needed to start the engine. It must also supply power to a large number of different electrical consumers. Convenience elements in particular (the air conditioning system, for example) but also safety systems such as the ABS and ESP have additional energy requirements which are not supported by the output of the alternator alone. This is particularly relevant given that standing traffic is an increasingly common sight in city centres, reducing the output of the alternator as a result.
Battery in use
New drive systems such as start/stop and hybrid vehicles are also placing new requirements on the performance and reliability of a modern starter battery. Similarly, starter batteries for trucks and HGVs have to meet specific requirements. They need to demonstrate particularly high vibration and cycle resistance. With these considerations in mind, modern AGM (Absorbent Glass Mat) batteries are at a significant advantage. In this type of battery, the electrolyte is bound in an absorbent glass fleece. This technology prevents acid layering and ensures very high vibration and cycle resistance at maximum output.
Design and technology
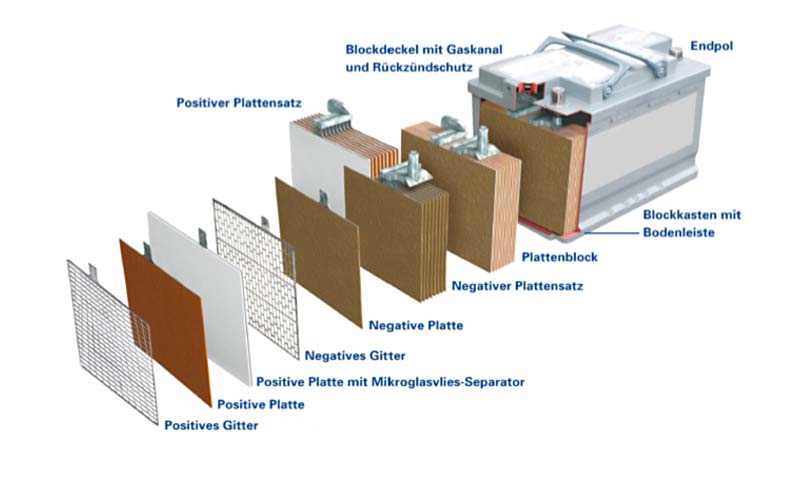
When the battery is connected to a load, a current flows, discharging the battery. The electrons move from the negative plate to the positive plate. This is offset by sulphate ions moving from the electrolyte to the negative plate, where they combine with the lead to form lead sulphate. Lead sulphate – with formation of water – is also produced from the lead dioxide at the positive plate when sulphate and hydrogen ions are consumed.
For charging, the battery is connected to a DC voltage source. The electrons flow from the positive plate to the negative plate. At the negative plate, the flow of electrons reduces the lead sulphate. At the positive plate, a process involving the release of electrons and the absorption of oxygen atoms turns the lead sulphate into lead dioxide. Sulphuric acid forms in the fluid and the amount of water is reduced.
Processes
In order for a lead-acid battery to emit current, the positive mass (lead dioxide) and the negative mass (lead) must be in direct contact with diluted sulphuric acid. The cell is the smallest unit in a battery. It contains positive and negative plates which are divided by what are known as separators (isolators). The more plate volume the cell has, the larger its capacity (in other words, the larger the plate volume, the more electricity the cell can emit).
The cell also contains diluted sulphuric acid. This acid penetrates the plates and separators, filling the cavities so that lead-oxide or lead particles are constantly in direct contact with acid. Therefore, some of the acid poured into the cell is to be found in the plates and separators and some of it outside the plates. The acid outside the plates acts as reserve acid and of course also helps to conduct the current inside the cell.
Depreciation
Correct maintenance and care is essential in order to maximise battery service life. This includes keeping the battery clean and dry at all times. Batteries that are not sealed should be checked regularly for acid level and if necessary topped up with distilled water. "Enhancing agents" must not be used. If the acid density falls below 1.21 kg/l, the battery must be recharged.
None of this is necessary for sealed batteries, as water consumption is significantly lower and checking the acid density and topping up with water are neither possible nor required.
If the battery is to be taken out of service for a prolonged period, it must be charged and stored upright in a cool and dry place. If a battery is taken out of use but left in the vehicle, the negative terminal must be disconnected. The protective cap should also not be removed from the positive pole. The state of charge must be checked regularly and if necessary corrected by means of recharging.
During charging, there must be sufficient room ventilation and only suitable DC units may be used. The battery's positive pole must be connected to the charger's positive output. The same rule applies for the negative connection. The charger is not switched on until after the positive and negative connections have been made. The recommended charge current is: 1/10 amps of the battery capacity (Ah). Charging should be aborted if the acid temperature exceeds 55°C. The battery is fully charged if the acid density and the charging voltage remain at the same level for 2 hours.
Battery storage
If the battery is to be decommissioned due to prolonged non-use, it must be charged, stored upright, cool and dry. If it remains in the vehicle, the negative terminal should be disconnected. In addition, the protective cap should be left on the positive terminal. The state of charge must be checked regularly and, if necessary, corrected by recharging.
Battery charging
If the battery is charged, care must be taken to ensure that the room is well ventilated. In addition, only suitable DC devices may be used. When charging the battery, the positive terminal of the battery must be connected to the positive output of the charger. When connecting the negative terminal, the same principle must be followed. Only after everything is connected together, the charger is switched on.
The charging current recommendation is 1/10 ampere (basic unit of electrical current) of the battery capacity (Ah). If the acid temperature exceeds 55 °C, charging should be interrupted. The battery is fully charged when the acid density and the charging voltage no longer increase within two hours.
Safety
Although modern starter batteries are designed with safety in mind, there are a number of things to consider in order to ensure optimum safety. For installation, it is vital that the battery is mounted securely.
Degassing holes must not be covered or heavily soiled. Under certain charging conditions, lead-acid batteries will generate a gas mixture that may be more or less explosive. Therefore, sufficient ventilation is vital during battery charging. The battery must never be operated in enclosed spaces. Age-worn batteries should be replaced in good time as gassing increases significantly as batteries get older.
Before batteries are installed or removed, all loads must be shut down to prevent the risk of sparking. When unplugging the connections, the earth terminal must be removed first. When plugging the connections back in, the earth terminal must be connected last. This prevents the risk of short-circuits caused by tools.
Sealed batteries (without plugs) should never be opened. It is not even necessary to do this, as these batteries are maintenance-free and therefore consume little water.
Series or parallel circuits must have identical layouts and be the same age. All batteries must have the same state of charge and the manufacturer's instructions must be followed precisely. Batteries must not be tilted more than 45° unless they are marked as tilt-proof and leak-proof.
Battery installation and removal
Degassing holes must not be covered or heavily soiled. Under certain charging conditions, lead-acid batteries will generate a gas mixture that may be more or less explosive. Therefore, sufficient ventilation is vital during battery charging. The battery must never be operated in enclosed spaces. Age-worn batteries should be replaced in good time as gassing increases significantly as batteries get older.
Before batteries are installed or removed, all loads must be shut down to prevent the risk of sparking. When unplugging the connections, the earth terminal must be removed first. When plugging the connections back in, the earth terminal must be connected last. This prevents the risk of short-circuits caused by tools.
Sealed batteries
Sealed batteries (without plugs) should never be opened. It is not even necessary to do this, as these batteries are maintenance-free and therefore consume little water.
Series and parallel circuits
Series or parallel circuits must have identical layouts and be the same age. All batteries must have the same state of charge and the manufacturer's instructions must be followed precisely. Batteries must not be tilted more than 45° unless they are marked as tilt-proof and leak-proof.
Battery storage
Series or parallel circuits must have identical layouts and be the same age. All batteries must have the same state of charge and the manufacturer's instructions must be followed precisely. Batteries must not be tilted more than 45° unless they are marked as tilt-proof and leak-proof.
Environmental protection
The European Union's new Batteries Directive came into force on 1 December 2009. All companies that produce, import and thus trade in batteries, or companies that install them, are affected by the Directive. Both the correct and qualified marking of batteries and the environmentally-friendly and sustainable disposal of spent batteries are key points of the legislation. Neither batteries nor accumulators should be simply released into the environment; they must be collected and disposed of properly. The Battery Directive sets out mandatory requirements for manufacturers governing not only the use of hazardous materials in production – cadmium in particular – but also volume collected and take-back rates. All batteries must be marked uniformly in such a way that it is immediately clear that they contain hazardous materials and do not belong with household waste. This is indicated by the crossed-out wheelie bin and the letters Pb (lead). Spent vehicle batteries must therefore be taken to dealers or garages for disposal in the correct and proper way. Lead-acid batteries can be recycled very easily. The raw materials obtained from this process are used in the production of new batteries.




